Another one down, every point in the spec should now be covered!
Good luck to all exam takers!
This blog will cover and explain the specification for Edexcel triple science course 2013 Chemistry. Hope it helps :)
Thursday 16 May 2013
1.30 recall the charges of common ions in this specification
K +
Na +
Li +
H +
Mg 2+
Ca 2+
Al 3+
Cl-
Br-
I-
F-
OH-
NO3-
SO4 2-
CO3 2-
Na +
Li +
H +
Mg 2+
Ca 2+
Al 3+
Cl-
Br-
I-
F-
OH-
NO3-
SO4 2-
CO3 2-
5.31 describe important uses of sodium hydroxide, including the manufacture of bleach, paper and soap; and of chlorine, including sterilising water supplies and in the manufacture of bleach and hydrochloric acid.
Sodium Hydroxide: Bleach; paper; soap
Chlorine: sterilising water; bleach; hydrochloric acid
5.29 describe the manufacture of sodium hydroxide and chlorine by the electrolysis of concentrated sodium chloride solution (brine) in a diaphragm cell
Brine is NaCl solution.
In electrolysis, chlorine is created at the anode, hydrogen is created at the cathode, sodium hydroxide is left in the solution.
2NaCl + 2H2O > 2NaOH + H2 + Cl2
This happens in a diaphragm cell.
In electrolysis, chlorine is created at the anode, hydrogen is created at the cathode, sodium hydroxide is left in the solution.
2NaCl + 2H2O > 2NaOH + H2 + Cl2
This happens in a diaphragm cell.
5.28 describe the use of sulfuric acid in the manufacture of detergents, fertilisers and paints
Detergents: used to 'sulphonate' products (apperently.)
Fertilisers: reacted to make phosphates soluble to plants: reacted to make ammonia easier to handle.
Paints: reacted with titanium ore to make a main pigment in paint.
http://bscstriplescience.wikispaces.com/file/view/C3_4_12+Why+is+sulphuric+acid+important.pdf
Fertilisers: reacted to make phosphates soluble to plants: reacted to make ammonia easier to handle.
Paints: reacted with titanium ore to make a main pigment in paint.
http://bscstriplescience.wikispaces.com/file/view/C3_4_12+Why+is+sulphuric+acid+important.pdf
5.27 describe the manufacture of sulfuric acid by the contact process, including the essential conditions
(stage 1) S + O2 > SO2
(stage 2) SO2 + O > SO3
(satge 3) SO3 + H2O > H2SO4
i) a temperature of about 450C
(stage 2) SO2 + O > SO3
(satge 3) SO3 + H2O > H2SO4
 |
| freewebs |
i) a temperature of about 450C
ii) a pressure of about 2 atmospheres
iii) a vanadium(V) oxide catalyst (in stage 2)
Very helpful source:
http://namedorganicreactions.co.uk/Sulphuric.pdf
Very helpful source:
http://namedorganicreactions.co.uk/Sulphuric.pdf
5.26 recall the raw materials used in the manufacture of sulfuric acid
Sulphur (sulphur is found in rocks and some natural gasses) and oxygen from the air and water.
5.25 describe the use of ammonia in the manufacture of nitric acid and fertilisers
Ammonia is put into fertilisers it contains nitrate ions plants need to make amino acids and so proteins plants need to grow.
Ammonia is also reacted with oxygen to produce nitric acid:
4NH3 + 8O2 > 4HNO3 + 4H2O
Ammonia is also reacted with oxygen to produce nitric acid:
4NH3 + 8O2 > 4HNO3 + 4H2O
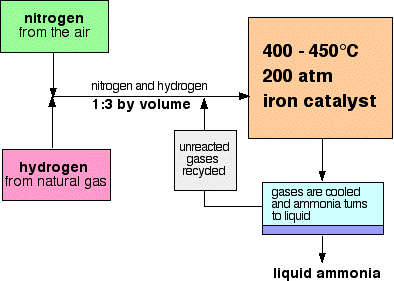
5.24 understand how the cooling of the reaction mixture liquefies the ammonia produced and allows the unused hydrogen and nitrogen to be recirculated
The products from the reactant are sent through a cooling mechanism, this is at a temperature that condenses ammonia, but not hydrogen and nitrogen. Liquid ammonia is then collected but hydrogen and nitrogen float right back into the reactor.
 |
| chemguide |
5.23 describe the manufacture of ammonia by the Haber process, including the essential conditions
5.22 understand that nitrogen from air, and hydrogen from natural gas or the cracking of hydrocarbons, are used in the manufacture of ammonia
Ammonia is made by reacting nitrogen from the air and hydrogen (which comes as a natural gas or from cracking hydrocarbons.)
5.21 understand that condensation polymerisation produces a small molecule, such as water, as well as the polymer
Two monomers come together by loosing a molecule. Atoms from each monomer join together to make the molecule: commonly a H atom from one and a OH molecule from another form water. The two monomers then join together, making a polymer.
5.20 understand that some polymers, such as nylon, form by a different process called condensation polymerisation
Two monomers join together when they loose a small molecule made up of atoms from both monomers.
Commonly, an H atom from one and and OH molecule from the other form water (hence condensation reaction) and the two monomers become joined.
Commonly, an H atom from one and and OH molecule from the other form water (hence condensation reaction) and the two monomers become joined.
 |
| askmichelle |
This is what happens to make nylon:
 |
| chemguide |
5.19 explain that addition polymers are hard to dispose of as their inertness means that they do not easily biodegrade
Polymers are saturated so they don't react. This means they don't decompose easily.
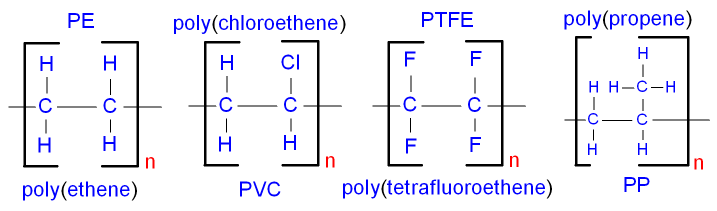
5.18 describe some uses for polymers, including poly(ethene), poly(propene) and poly(chloroethene)
polyethene: plastic carrier bags; plastic bottels
polypropene: crates; ropes
polychlroethene: piping; cable insulation.
polypropene: crates; ropes
polychlroethene: piping; cable insulation.
5.17 deduce the structure of a monomer from the repeat unit of an addition polymer
A monomer that is repeated in a polymer looks much like the repeat unit; apart from, instead of having an empty bond either end, it has a double bond in the middle.
.gif) |
| askmichelle |
5.16 draw the repeat unit of addition polymers, including poly(ethene), poly(propene) and poly(chloroethene)
The repeat unit is the structure that is repeated to form a polymer.
(ignore PTFE on this diagram)
(ignore PTFE on this diagram)
 |
| GCSEscience |
5.15 understand that an addition polymer is formed by joining up many small molecules called monomers
monomers are alkenes with a double bond. If this bond is broken there can be other things bonded, if a carbon from another monomer is bonded in then you can create a chain; do this many times can you have a polymer.
5.14 describe how long-chain alkanes are converted to alkenes and shorter-chain alkanes by catalytic cracking, using silica or alumina as the catalyst and a temperature in the range of 600–700C.
Long chain hydrocarbons are passed over a hot catalyst (silica or alumina at 600-700 degrees) this causes them to break down into smaller molecules.
As some atoms are lost from molecules, they become unsaturated and can therefore form a double bond. This is how you get alkenes from the process as well as shorter chain alkanes.
The animation on this page is helpful:
http://www.bbc.co.uk/schools/gcsebitesize/science/aqa_pre_2011/oils/polymersrev1.shtml
As some atoms are lost from molecules, they become unsaturated and can therefore form a double bond. This is how you get alkenes from the process as well as shorter chain alkanes.
The animation on this page is helpful:
http://www.bbc.co.uk/schools/gcsebitesize/science/aqa_pre_2011/oils/polymersrev1.shtml
5.13 understand that fractional distillation of crude oil produces more long-chain hydrocarbons than can be used directly and fewer short-chain hydrocarbons than required and explain why this makes cracking necessary
Long chain hydrocarbons are less flammable and more viscous.
Short chain hydrocarbons burn well and flow well.
Long chain hydrocarbons can be cracked which breaks them up into short chain ones.
Short chain hydrocarbons burn well and flow well.
Long chain hydrocarbons can be cracked which breaks them up into short chain ones.
5.12 understand that nitrogen oxides and sulfur dioxide are pollutant gases which contribute to acid rain, and describe the problems caused by acid rain
NO and SO2 are given off into the atmosphere by some industrial processes.
When they are in the atmosphere they react with rain water to create H+ ions.
When the rain falls the acid can corrode rocks and buildings. Acid can also alter the PH in soil or rivers which can effect an ecosystem.
Also acid rain corrodes limestone, which damages buildings and stuff.
When they are in the atmosphere they react with rain water to create H+ ions.
When the rain falls the acid can corrode rocks and buildings. Acid can also alter the PH in soil or rivers which can effect an ecosystem.
Also acid rain corrodes limestone, which damages buildings and stuff.
5.11 understand that, in car engines, the temperature reached is high enough to allow nitrogen and oxygen from air to react, forming nitrogen oxides
In car engines there is a high enough temperature to cause a reaction between oxygen and nitrogen in the air.
This makes NO.
This makes NO.
5.10 understand that incomplete combustion of fuels may produce carbon monoxide and explain that carbon monoxide is poisonous because it reduces the capacity of the blood to carry oxygen
Hydrocarbons (from crude oil) + oxygen > carbon dioxide + water
Unless there is not enough oxygen around. Then fuels will combust incompletely:
Hydrocarbons + oxygen > carbon monoxide + carbon + water
Carbon monoxide combines with heamaglobin in red blood cells, meaning they can't carry oxygen around the body.
Unless there is not enough oxygen around. Then fuels will combust incompletely:
Hydrocarbons + oxygen > carbon monoxide + carbon + water
Carbon monoxide combines with heamaglobin in red blood cells, meaning they can't carry oxygen around the body.
5.9 describe the trend in boiling point and viscosity of the main fractions
Fractions with low boiling points are less viscous.
Fractions with high boiling points are more viscous.
Fractions with high boiling points are more viscous.
5.7 describe and explain how the industrial process of fractional distillation separates crude oil into fractions
Crude oil is heated until it boils. As a gas it floats upwards.
As the gas goes higher up and further from the heat source the temperature decreases.
When a compound reaches it condensing point it will condense into a liquid and be collected.
This is known as fractional distillation, and groups with similar condensing temperatures are known as fractions; each fraction is a different substance.
As the gas goes higher up and further from the heat source the temperature decreases.
When a compound reaches it condensing point it will condense into a liquid and be collected.
This is known as fractional distillation, and groups with similar condensing temperatures are known as fractions; each fraction is a different substance.
5.6 understand that crude oil is a mixture of hydrocarbons
Crude oil contains different molecules made only of hydrogen and carbon.
5.5 explain the uses of aluminium and iron, in terms of their properties
Aluminium is 'low density' and does not corrode so is used for the bodies of planes.
Iron is used in electromagnets as it is a soft magnetic material.
Iron is used in electromagnets as it is a soft magnetic material.
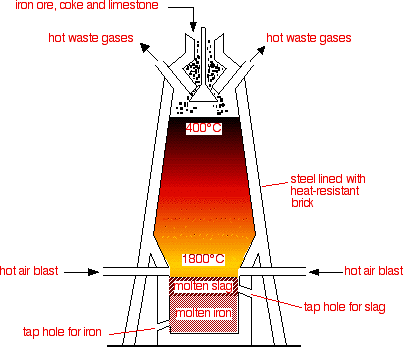
5.4 describe and explain the main reactions involved in the extraction of iron from iron ore (haematite), using coke, limestone and air in a blast furnace
Iron is displaced from its ore (haematite) by carbon (from coke):
2Fe2O3 + 3C > 4Fe + 3CO2
It is also displaced by carbon monoxide
Fe2O3 + 3CO > 2Fe + 3CO2
Limestone reacts with impurities to from 'slag' which is tapped off.
Air allows burning to take place (of coke.)
2Fe2O3 + 3C > 4Fe + 3CO2
It is also displaced by carbon monoxide
Fe2O3 + 3CO > 2Fe + 3CO2
Limestone reacts with impurities to from 'slag' which is tapped off.
Air allows burning to take place (of coke.)
 |
| chemguide |
5.3 write ionic half-equations for the reactions at the electrodes in aluminium extraction
Al3+ + 3e- > Al
- 2O2- → O2 + 4e-
5.2 describe and explain the extraction of aluminium from purified aluminium oxide by electrolysis
- Bauxite is purified into aluminium oxide.
- This is then dissolved in molten cryolite to bring down the boiling point
- The walls of the tank are the negative electrode; here aluminium is made
- The aluminium sinks to the bottom and is tapped off
- Oxygen is formed at the positive electrode
- The oxygen formed reacts with the graphite anode to from carbon dioxide; so the anode has to be replaced
5.1 explain how the methods of extraction of the metals in this section are related to their positions in the reactivity series
Anything below carbon can be displaced from its ore by carbon.
Anything above carbon can't so is extracted by electrolysis.
| frankswebspace |
4.9 describe experiments to carry out acid-alkali titrations
- Have a known volume of acid in a beaker with methyl orange, the solution will be red as it is very acidic.
- Set up a burette with alkali in it. Open the tap very slightly, so that it drips very slowly into the acid.
- With each drop stir the contents of the beaker.
- The more alkali that is added the more neutral and closer to orange it gets.
- When the solution is neutral the it will be completely orange, at this point close the tap on the burette.
- The level in the burette will have dropped, showing the volume of alkali used.
- This shows you how much alkali you need to use to neutralise the acid.
4.8 describe experiments to prepare insoluble salts using precipitation reactions
Silver nitrate and sodium chloride are added together, the product, silver chloride is made, this salt is insoluable and so will form a white precipitate in the solution.
AgNO3 + NaCl > AgCl + NaNO3
AgNO3 + NaCl > AgCl + NaNO3
4.7 describe experiments to prepare soluble salts from acids
Dilute sulphuric acid is added to an excess of magnesium.
Mg + H2SO4 > MgSO4 + H2
The left over magnesium is filtered off and the mixture boiled down slowly to concentrate.
When it is cooled, crystals will form, these can be blotted dry with a piece of paper.
Mg + H2SO4 > MgSO4 + H2
The left over magnesium is filtered off and the mixture boiled down slowly to concentrate.
When it is cooled, crystals will form, these can be blotted dry with a piece of paper.
This can be done with other metals and acids:
Nitric, sulphuric and hydrochloric acids make soluble salts with most metals.
ammonium, potassium and sodium make soluble salts with acids
4.25 predict the effects of changing the pressure and temperature on the equilibrium position in reversible reactions.
If you move the equilibrium, you change the rate of reaction.
If the equilibrium moves to the right, you have more products. (the reactants are reacting faster)
If the equilibrium moves to the left, you have more reactants. (the products are reacting faster)
If you increase the pressure: the equilibrium will move to the side with least molecules.
If you decrease the pressure: the equilibrium will move to the side with the most molecules.
If you increase the temperature, there will be more products that are produced by an endothermic reaction.
This is because the reaction is trying to use up the extra heat, and it does so by putting the energy into making bonds.
If the equilibrium moves to the right, you have more products. (the reactants are reacting faster)
If the equilibrium moves to the left, you have more reactants. (the products are reacting faster)
If you increase the pressure: the equilibrium will move to the side with least molecules.
If you decrease the pressure: the equilibrium will move to the side with the most molecules.
If you increase the temperature, there will be more products that are produced by an endothermic reaction.
This is because the reaction is trying to use up the extra heat, and it does so by putting the energy into making bonds.
4.24 understand the concept of dynamic equilibrium
Dynamic equilibrium is when a reversible reaction is happening both ways at the same time, at the same rate.
4.23 describe reversible reactions such as the dehydration of hydrated copper(II) sulfate and the effect of heat on ammonium chloride
If you add water to copper sulphate you can make hydrated copper sulphate.
If you remover the water from hydrated copper sulphate you can make copper sulphate.
When heated, ammonium chloride splits into to hydrogen chloride and ammonia.
Hydrogen chloride and ammonia can be reacted to make ammonium chloride.
If you remover the water from hydrated copper sulphate you can make copper sulphate.
When heated, ammonium chloride splits into to hydrogen chloride and ammonia.
Hydrogen chloride and ammonia can be reacted to make ammonium chloride.
4.22 understand that some reactions are reversible and are indicated by the symbol ⇌ in equations
Some reactions can happen both ways: the reactants can make the products and the products can make the reactants.
This symbol shows that ⇌.
This symbol shows that ⇌.
4.21 explain that a catalyst speeds up a reaction by providing an alternative pathway with lower activation energy
A catalyst provides an alternative route for the reaction to start, this route requires less energy to start the reaction.
4.18 describe the effects of changes in surface area of a solid, concentration of solutions, pressure of gases, temperature and the use of a catalyst on the rate of a reaction
Higher temperature, more surface area, higher concentration, higher pressure and use of a catalyst all make a reaction faster.
4.19 understand the term activation energy and represent it on a reaction profile
Activation energy is the amount of energy required for a reaction start happening.
| chemguide |
4.20 explain the effects of changes in surface area of a solid, concentration of solutions, pressure of gases and temperature on the rate of a reaction in terms of particle collision theory
Collision theory says that to react particles must:
Collide with enough energy to react
Collide in the right orientation to react (the more frequent the collisions, the more likely this is)
Surface area
Concentration/ pressure
Temperature
Catalyst
Collide with enough energy to react
Collide in the right orientation to react (the more frequent the collisions, the more likely this is)
Surface area
- Particles collide more frequently if there is more surface area, as there is more contact between the reactants. Faster rate of reaction.
Concentration/ pressure
- There is more chance of particles colliding at a higher concentration/pressure, so they react more often. Faster rate of reaction.
Temperature
- Particles move about more and will collide more frequently the higher the temperature; react more often. Increases the rate of reaction.
Catalyst
- Provides an alternative pathway for the reaction to start which requires a lower activation energy.
4.17 describe experiments to investigate the effects of changes in surface area of a solid, concentration of solutions, temperature and the use of a catalyst on the rate of a reaction
Surface area
Concentration
Temperature
Catalyst
- Put a set mass of magnesium in hydrochloric acid
- Time the reaction
- Change the from of magnesium keeping the mass the same (powder, wire, strips)
- The more surface area (the smaller the pieces of magnesium) the faster the reaction
Concentration
- Put a set mass of marble chips into dilute hydrochloric acid
- Time the reaction
- Change the ratio of water to hydrochloric acid
- The more concentrated the hydrochloric acid (the lower the ratio of water) the faster the reaction
Temperature
- Put a set mass of magnesium powder into a set mass of hydrochloric acid
- time the reaction
- Carry out this reaction at different temperatures
- The higher the temperature the faster the rate of reaction
Catalyst
- If you have hydrogen peroxide it will not decompose
- If you put it with manganese dioxide it will decompose into water and oxygen
- The manganese dioxide will be unaltered by the reaction
- The more of the catalyst the faster the reaction
4.16 use average bond energies to calculate the enthalpy change during a simple chemical reaction.
To work out the enthalpy change you do:
the sum of bond energies in the reactants - the sum of bond energies in the products
Given average bond energies, you can easily work out the sum of bond energies.
For example, if we are told that
C-H = 413
O=O= 498
C=O= 746
H-O=464
Work out the enthalpy change for this reaction: CH4 + 2O2 > CO2 + 2H2O
((4 x C-H) + (2 x O=O)) - ((2x C=O) + (4 x H-O))
Then we substitute in:
(4 x 413) + (2 x 498) - (2 x 746) + (4 x 464) = 2648KJ - 3348KJ = 700KJ enthalpy change
Credit to author of this source: https://www.google.co.uk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=5&cad=rja&ved=0CFoQFjAE&url=http%3A%2F%2Fchemistryatdulwich.wikispaces.com%2Ffile%2Fview%2FSection%2B4%2Bb%2Benergetics.doc%2F183320535%2FSection%25204%2520b%2520energetics.doc&ei=a72UUZOaHYa_0QXMkoD4Bg&usg=AFQjCNF9yn2bAWyGOPYSO4W1NrJExx7HQw&sig2=_XxB8HEYh8MHVUe5abc6sA&bvm=bv.46471029,d.d2k
the sum of bond energies in the reactants - the sum of bond energies in the products
Given average bond energies, you can easily work out the sum of bond energies.
For example, if we are told that
C-H = 413
O=O= 498
C=O= 746
H-O=464
Work out the enthalpy change for this reaction: CH4 + 2O2 > CO2 + 2H2O
((4 x C-H) + (2 x O=O)) - ((2x C=O) + (4 x H-O))
Then we substitute in:
(4 x 413) + (2 x 498) - (2 x 746) + (4 x 464) = 2648KJ - 3348KJ = 700KJ enthalpy change
Credit to author of this source: https://www.google.co.uk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=5&cad=rja&ved=0CFoQFjAE&url=http%3A%2F%2Fchemistryatdulwich.wikispaces.com%2Ffile%2Fview%2FSection%2B4%2Bb%2Benergetics.doc%2F183320535%2FSection%25204%2520b%2520energetics.doc&ei=a72UUZOaHYa_0QXMkoD4Bg&usg=AFQjCNF9yn2bAWyGOPYSO4W1NrJExx7HQw&sig2=_XxB8HEYh8MHVUe5abc6sA&bvm=bv.46471029,d.d2k
4.15 understand that the breaking of bonds is endothermic and that the making of bonds is exothermic
Breaking bonds requires energy, this takes in heat: endothermic.
Making bonds releases energy, this gives out heat: exothermic.
Making bonds releases energy, this gives out heat: exothermic.
4.14 represent exothermic and endothermic reactions on a simple energy level diagram
Exothermic: lower energy level at end;
Endothermic: more energy at end;
Activation energy is the energy requires to start the reaction.
| gcsescience |
| docbrown |
4.13 understand the use of ΔH to represent enthalpy change for exothermic and endothermic reactions
ΔH is the symbol that represents the amount of energy lost or gained in a reaction.
+ΔH is endothermic (because it gains heat)
-ΔH is exothermic (because it looses heat)
+ΔH is endothermic (because it gains heat)
-ΔH is exothermic (because it looses heat)
4.12 calculate molar enthalpy change from heat energy change
|
ΔH
=
|
T
(C)
x mass of H2O
(g) x 4.2J/g C./
|
|
number
of moles
|
ΔH
is measured in J/mol or
kJ/mol.
- ΔH = molar enthalpy change
- T = temperature increase or decrease caused by the reaction
- mass of H2O = mass of water in grams;
- 4.2J/g C = specific heat capacity of water
4.11 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation in which heat energy changes can be calculated from measured temperature changes
Measure the temperature at the beginning of the experiment, measure the temperature at the end. How ever much heat has gone up or down is the calorimetry of the reaction.
For example if you have a beaker of water and take its temperature, then burn a piece of bread under it, the change in temperature is the calories (energy) of the bread.
For example if you have a beaker of water and take its temperature, then burn a piece of bread under it, the change in temperature is the calories (energy) of the bread.
4.10 understand that chemical reactions in which heat energy is given out are described as exothermic and those in which heat energy is taken in are endothermic
In an exothermic reaction heat is given out. Because bonds are made which gives out energy.
Think expelled; exit; exo.
In a endothermic reaction heat is taken in. Because bonds are Broken which requires energy.
Think expelled; exit; exo.
In a endothermic reaction heat is taken in. Because bonds are Broken which requires energy.
4.6 understand the general rules for predicting the solubility of salts in water
i) all common sodium, potassium and ammonium salts are soluble
ii) all nitrates are soluble
iii) common chlorides are soluble, except silver chloride
iv) common sulfates are soluble, except those of barium and calcium
v) common carbonates are insoluble, except those of sodium, potassium and
ammonium
4.5 predict the products of reactions between dilute hydrochloric, nitric and sulfuric acids; and metals, metal oxides and metal carbonates
Metals
Metal oxides
Metal carbonates
- Hydrochloric acid + metal > metal chloride salt + hydrogen
- Nitric acid + metal > You don't need to know
- Sulphuric acid + metal > metal sulphate + hydrogen
Metal oxides
- Hydrochloric acid + metal oxide > metal chloride salt + water
- Nitric acid + metal oxide > metal nitrate salt + water
- Sulphuric acid + metal oxide > metal sulphate + water
Metal carbonates
- Hydrochloric acid + metal carbonate > metal chloride salt + water + carbon dioxide
- Nitric acid + metal carbonate > metal nitrate salt + water + carbon dioxide
- Sulphuric acid + metal carbonate > metal sulphate + water + carbon dioxide
4.4 define acids as sources of hydrogen ions, H+, and alkalis as sources of hydroxide ions, OH¯
Essentially if something is acidic it contains positive hydrogen ions, if something is alkaline it contains negative hydroxide ions.
4.3 describe the use of universal indicator to measure the approximate pH value of a solution

Neutral is green
The more red, the more acidic.
The more purple, the more alkaline.
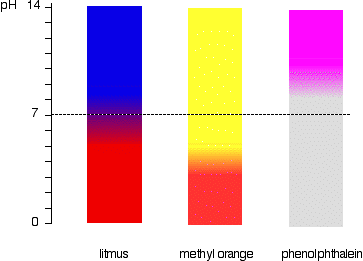
4.1 describe the use of the indicators litmus, phenolphthalein and methyl orange to distinguish between acidic and alkaline solutions
Red litmus paper turns blue in the presence of alkali.
Blue litmus paper turns red in the presence of acid.
Phenolphthalein goes pink in alkalis.
Methyl orange is yellow for alkali, red for acid.
Blue litmus paper turns red in the presence of acid.
Phenolphthalein goes pink in alkalis.
Methyl orange is yellow for alkali, red for acid.
 |
| chemguide |
3.12 describe the dehydration of ethanol to ethene, using aluminium oxide.
C2H5OH > C2H4 + H2O
ethanol > ethene + water
aluminium oxide is the catalyst for this reaction.
ethanol > ethene + water
aluminium oxide is the catalyst for this reaction.
3.11 evaluate the factors relevant to the choice of method used in the manufacture of ethanol, for example the relative availability of sugar cane and crude oil
Fermentation
Hydrating (ethene and steam)
- Cane sugar widely avalible/ cheap/ renewable
- Slow process
- Impurities in the product
- Done in batches
Hydrating (ethene and steam)
- Crude oil (cracked to make ethene) expensive/ non-renewable
- Fast process
- Pure product
- Continuous reaction
3.10 describe the manufacture of ethanol by the fermentation of sugars, for example glucose, at a temperature of about 30°C
Ethanol can be made by the anaerobic respiration of microorganisms.
glucose > ethanol + carbon dioxide
This happens at 30 degrees.
glucose > ethanol + carbon dioxide
This happens at 30 degrees.
3.9 describe the manufacture of ethanol by passing ethene and steam over a phosphoric acid catalyst at a temperature of about 300°C and a pressure of about 60–70 atm
C2H4 (ethene) + H2O (steam) > C2H5OH (ethanol)
This reaction takes place at a high pressure (60-70 atm) and a high temperature (300) to make the reaction happen very quickly. Phosphoric acid also speeds up the reaction as it is a catalyst.
This reaction takes place at a high pressure (60-70 atm) and a high temperature (300) to make the reaction happen very quickly. Phosphoric acid also speeds up the reaction as it is a catalyst.
2.39 describe tests for the gases
i
hydrogen
- burns with a 'squeaky pop' sound
ii
oxygen
- will relight a glowing splint
iii
carbon dioxide
- Turns lime water cloudy
iv
ammonia
- Damp red litmus paper blue
- Damp universal indicator purple
v
chlorine
- bleaches damp litmus paper white
2.38 describe tests for the anions
i) Cl-,
Br- and
I-,
using dilute nitric acid and silver nitrate solution
- Chloride ions + nitric acid + silver nitrate > white precipitate (silver chloride)
- Bromide ions + nitric acid + silver nitrate > cream precipitate (silver bromide)
- Iodide ions + nitric acid + silver nitrate > yellow precipitate (silver iodide)
- SO4(2-) + HCl + Ba(2+) > white precipitate (barium sulphate)
iii) CO3 2-,
using dilute hydrochloric acid and identifying the carbon dioxide evolved
- Carbonate + acid > salt + water + carbon dioxide
- Carbon dioxide produced will turn lime water cloudy
2.37 describe tests for the cations
i) Li+,
Na+,
K+,
Ca2+
using
flame tests
- Lithum: red
- Sodium: orange (so strong can mask other colours)
- Potassium: lilac
- Calcium: brick red
ii) NH4+,
using sodium hydroxide solution and identifying the ammonia evolved
- NH4 + OH > NH3 + H2O
- ammonium ions + hydroxide ions > ammonia + water
- ammonia (pungent smelling gas) turns red litmus paper blue
iii) Cu2+,
Fe2+
and
Fe3+,
using sodium hydroxide solution
- Copper(ii) sulphate + sodium hydroxide > blue precipitate
- Iron(ii) sulphate + sodium hydroxide > green precipitate
- Iron(iii) sulphate + sodium hydroxide > brown precipitate
Wednesday 15 May 2013
3.8 describe the addition reaction of alkenes with bromine, including the decolourising of bromine water as a test for alkenes
An alkene will make its double bond into a single bond, to bond to two bromines. Bromine is added to the molecule. The product made is colourless. When alkenes are put in bromine water it turns from brown to colourless (a good way of testing for alkenes.)
For example:
C2H4(g) + Br2 (aq) → C2H4Br2 (aq)
For example:
C2H4(g) + Br2 (aq) → C2H4Br2 (aq)
3.7 draw displayed formulae for alkenes with up to four carbon atoms in a molecule, and name the straight-chain isomers
In every alkene there is one double bond between two carbons. Bearing in mind that carbons can only make four bonds, then the double bonded carbons will either be joined to: another carbon and a hydrogen; or two hydrogens.
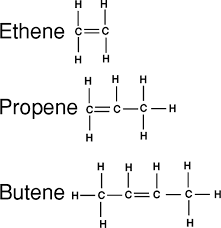
3.6 recall that alkenes have the general formula CnH2n
All compounds in the homologous group alkenes have the general formula CnH2n.
3.5 describe the substitution reaction of methane with bromine to form bromomethane in the presence of UV light.
In UV light bromine and methane will form bromomethane:
CH4 + Br2 CH3Br + HBr
CH3Br + HBr
What has happened in this reaction is a bromine has taken the place of a hydrogen (substitution.)
CH4 + Br2
3.4 recall the products of the complete and incomplete combustion of alkanes
Complete combustion gives carbon dioxide and water.
Incomplete combustion gives carbon monoxide and water.
Incomplete combustion gives carbon monoxide and water.
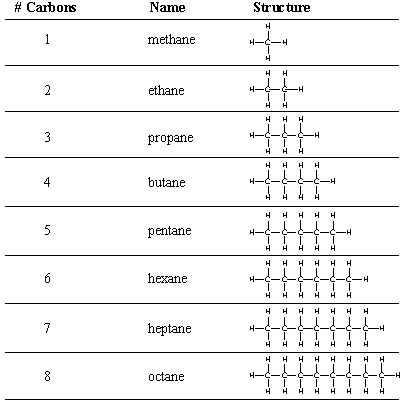
3.3 draw displayed formulae for alkanes with up to five carbon atoms in a molecule, and name the straight-chain isomers
The formula for alkanes is CnH2n+2. This means that every carbon is bonded to two hydrogens, and there is also one hydrogen on each end.
The isomers you are likely to encounter will just be this formula in a straight line (you only need up to pentane):
The isomers you are likely to encounter will just be this formula in a straight line (you only need up to pentane):
 |
| sparknotes |
2.36 understand the sacrificial protection of iron in terms of the reactivity series.
Sacrificial is covering a metal with a more reactive metal. What this means is water and/or air will react with the more reactive metal instead of the one underneath.
2.35 describe how the rusting of iron may be prevented by grease, oil, paint, plastic and galvanising
Grease, oil, paint and plastic prevent air and/or water from coming into contact with iron. This means the reaction that rusts iron can't occur.
Galvanising is coating in zinc. This Zinc react in the air to form ZnCO3 which prevents air and/or water from coming into contact with the iron.
Galvanising is coating in zinc. This Zinc react in the air to form ZnCO3 which prevents air and/or water from coming into contact with the iron.
3.2 recall that alkanes have the general formula CnH2n +2
Alkanes is a homologous series with the formula CnH2n +2.
What this means is that for every one carbon there are two times the amount of hydrogens plus two more hydrogens.
What this means is that for every one carbon there are two times the amount of hydrogens plus two more hydrogens.
3.1 explain the terms homologous series, hydrocarbon, saturated, unsaturated, general formula and isomerism.
Compounds in the same homologous series have the same general formula and similar chemical properties.
A hydrocarbon is a compound made up only of hydrogen and carbon.
Saturated means something has bonded as many time as possible. Unsaturated means that more bonds can be made.
General formula is the most simplified the ratio of molecules can be.
Isomers have the same general formula but different structures.
A hydrocarbon is a compound made up only of hydrogen and carbon.
Saturated means something has bonded as many time as possible. Unsaturated means that more bonds can be made.
General formula is the most simplified the ratio of molecules can be.
Isomers have the same general formula but different structures.
2.34 describe the conditions under which iron rusts
Water and oxygen are needed to rust iron: iron that reacts with these becomes hydrated iron(iii) oxide.
2.33 understand the terms redox, oxidising agent, reducing agent
In a redox reaction, a more reactive metal gains an oxygen from a less reactive metal which looses it.
i.e. a more reactive metal is oxidised and a less reactive metal is reduced.
The reducing agent is the more reactive metal which reduces the other metal.
The oxidising agent is the less reactive metal which allows the other metal to be oxidised.
i.e. a more reactive metal is oxidised and a less reactive metal is reduced.
The reducing agent is the more reactive metal which reduces the other metal.
The oxidising agent is the less reactive metal which allows the other metal to be oxidised.
2.32 understand oxidation and reduction as the addition and removal of oxygen respectively
oxidation is the gain of oxygen,
reduction is the loss of oxygen.
reduction is the loss of oxygen.
2.31 deduce the position of a metal within the reactivity series using displacement reactions between metals and their oxides, and between metals and their salts in aqueous solutions
A metal oxide or a metal salt dissolved in water:
- introduce a more reactive metal and it will displace the current one
- introduce a less reactive metal and no displacement will take place
2.30 describe how reactions with water and dilute acids can be used to deduce the following order of reactivity: potassium, sodium, lithium, calcium, magnesium, zinc, iron and copper
potassium, sodium, lithium and calcium all react with water and acids
magnesium, zinc and iron all react with acids (and very slowly with water.)
copper doesn't react with either.
The more vigorous the reaction the more reactive the metal. The more things a metal will react with, the more reactive the metal.
magnesium, zinc and iron all react with acids (and very slowly with water.)
copper doesn't react with either.
The more vigorous the reaction the more reactive the metal. The more things a metal will react with, the more reactive the metal.
Tuesday 7 May 2013
2.28 describe a physical test to show whether water is pure.
If water is pure it will boil at exactly 100° and freeze at exactly 0°
2.27 describe the use of anhydrous copper(II) sulfate in the chemical test for water
anhydrous copper sulphate will become hydrous copper sulphate when it is reacted with water.
So if anhydrous copper sulphate goes from white to blue in the presence of a liquid it will be water.
So if anhydrous copper sulphate goes from white to blue in the presence of a liquid it will be water.
2.25 describe the reactions of dilute hydrochloric and dilute sulfuric acids with magnesium, aluminium, zinc and iron
acid
+ metal > salt + hydrogen
For example:
magnesium + hydrochloric acid > magnesium chloride + Hydrogen
Mg + 2HCl > MgCl2 + H2
For example:
magnesium + hydrochloric acid > magnesium chloride + Hydrogen
Mg + 2HCl > MgCl2 + H2
2.26 describe the combustion of hydrogen
The combustion of hydrogen is its reaction with oxygen.
Water is created. and a lot of energy.
2H2 + O2 > 2H2O
Water is created. and a lot of energy.
2H2 + O2 > 2H2O
2.24 understand that carbon dioxide is a greenhouse gas and may contribute to climate change
Carbon dioxide prevents heat leaving the earth's atmosphere in rays that the earth emits.
Significant amounts of green house gasses will warm up the earth, changing the climate.
Significant amounts of green house gasses will warm up the earth, changing the climate.
2.23 explain the use of carbon dioxide in carbonating drinks and in fire extinguishers, in terms of its solubility and density
Carbon dioxide is dissolved into drinks at a high pressure, this makes CO2 bubbles in fizzy drinks.
Some fire extinguishers have CO2 in, because it is denser than air it will fall over the fire creating a barrier between the air and fire: the fire can't burn with out the oxygen in the air.
Some fire extinguishers have CO2 in, because it is denser than air it will fall over the fire creating a barrier between the air and fire: the fire can't burn with out the oxygen in the air.
2.22 describe the properties of carbon dioxide, limited to its solubility and density
It is denser than air.
It is soluble in water at a high pressure.
It is soluble in water at a high pressure.
2.21 describe the formation of carbon dioxide from the thermal decomposition of metal carbonates such as copper(II) carbonate
When metal carbonates are heated they become carbon dioxide and a metal.
For example:
copper carbonate > copper oxide + carbon dioxide
CuCO3 > CuO + CO2
For example:
copper carbonate > copper oxide + carbon dioxide
CuCO3 > CuO + CO2
2.20 describe the laboratory preparation of carbon dioxide from calcium carbonate and dilute hydrochloric acid
calcium carbonate + hydrochloric acid → calcium chloride + water + carbon dioxide
CaCO3 + 2HCl → CaCl2 + H2O + CO2
CaCO3 + 2HCl → CaCl2 + H2O + CO2
2.19 describe the reactions of magnesium, carbon and sulfur with oxygen in air, and the acid-base character of the oxides produced
The two non-metals burn in air- giving out heat and light- to bond with oxygen.
They become non-metal oxides, which are, by nature, acids.
Magnesium will burn in air to from a metal-oxide, these are always basic.
They become non-metal oxides, which are, by nature, acids.
Magnesium will burn in air to from a metal-oxide, these are always basic.
2.18 describe the laboratory preparation of oxygen from hydrogen peroxide, using manganese(IV) oxide as a catalyst
hydrogen peroxide is put in a conical flask with manganese(IV) oxide as a catalyst. Plus there could be some water to diloute the hydrogen peroxide.
Hydrogen peroxide > water + oxygen
2H2O2 > 2H2O + O2
You can then collect oxygen by downwards displacement method.
This video is helpful:
http://www.youtube.com/watch?v=nkeniDKGs6Q
Hydrogen peroxide > water + oxygen
2H2O2 > 2H2O + O2
You can then collect oxygen by downwards displacement method.
This video is helpful:
http://www.youtube.com/watch?v=nkeniDKGs6Q
2.17 explain how experiments involving the reactions of elements such as copper, iron and phosphorus with air can be used to investigate the percentage by volume of oxygen in air
Copper, iron and phosphorus all react with air.
If you know the volume of air that you have, then react it with on of these, then re measure the volume of air; what has been lost is all oxygen that reacted.
This page describes it well:
http://www.bbc.co.uk/schools/gcsebitesize/science/aqa_pre_2011/oils/changesrev5.shtml
If you know the volume of air that you have, then react it with on of these, then re measure the volume of air; what has been lost is all oxygen that reacted.
This page describes it well:
http://www.bbc.co.uk/schools/gcsebitesize/science/aqa_pre_2011/oils/changesrev5.shtml
2.15 understand these displacement reactions as redox reactions.
When a more reactive halogen displaces a less reactive one this is a redox reaction.
This means that one element has been reduced (gained electrons) and one has been oxidised (lost electrons.)
Helpful hint!
Oilrig helps to remind you how redox reactions go:
Oxidation
Is
Loss
Reduction
Is
Gain
2.14 describe experiments to demonstrate that a more reactive halogen will displace a less reactive halogen from a solution of one of its salts
A more reactive halogen will displace a less reactive one that is bonded as a salt. This will only happen if the salt is dissolved in water or a gas.
This page has a really good example:
http://www.bbc.co.uk/schools/gcsebitesize/science/edexcel_pre_2011/patterns/groupsrev5.shtml
This page has a really good example:
http://www.bbc.co.uk/schools/gcsebitesize/science/edexcel_pre_2011/patterns/groupsrev5.shtml
2.12 explain, in terms of dissociation, why hydrogen chloride is acidic in water but not in methylbenzene
Water (H2O) is a polar molecule.
Methylbenzene is a non-polar molecule.
Hydrogen chloride is a polar molecule.
Polars only dissolve in polars.
When hydrogen chloride is dissolved you get +H ions.
These are acidic.
Wednesday 1 May 2013
2.13 describe the relative reactivities of the elements in Group 7
Group 7 elements become less reactive as you go down the group.
At the top, the positive charge of the proton in the nucleus is close to the surface (as there are few shells) this makes it easy for them to pull in the one electron they need to become stable, meaning they are very reactive.
Lower down where there are more shells the pull of the proton is further from the surface making it less easy to pull in another electron.
At the top, the positive charge of the proton in the nucleus is close to the surface (as there are few shells) this makes it easy for them to pull in the one electron they need to become stable, meaning they are very reactive.
Lower down where there are more shells the pull of the proton is further from the surface making it less easy to pull in another electron.
2.11 understand the difference between hydrogen chloride gas and hydrochloric acid
Hydrogen chloride gas is HCl
Hydrochloric acid is hydrogen chloride dissolved in water. The two ions become detached leaving Cl- and H+ ions. H+ is acidic, hence the term acid.
Hydrochloric acid is hydrogen chloride dissolved in water. The two ions become detached leaving Cl- and H+ ions. H+ is acidic, hence the term acid.
2.10 make predictions about the properties of other halogens in this group
We would expect the colour to keep getting darker and the melting and boiling points to keep getting higher further down the group.
2.9 recall the colours and physical states of the elements at room temperature
Fluorine.............. Gas........... yellow
Chlorine............. Gas........... yellow-green
Bromine............. Liquid........ red-brown
Iodine................ Solid.......... purple
Astatine............. Solid.......... black
Chlorine............. Gas........... yellow-green
Bromine............. Liquid........ red-brown
Iodine................ Solid.......... purple
Astatine............. Solid.......... black
2.7 describe the relative reactivities of the elements in Group 1
Group one elements get more reactive the further down the group.
2.8 explain the relative reactivities of the elements in Group 1 in terms of distance between the outer electrons and the nucleus.
Group one elements are more reactive further down the group.
Group one elements need to loose an electron- the one on the outer shell- to react. Electrons are held to an atom by the protons in the nucleus. If an electron is close to the nucleus the force holding it in will be very strong, if it is further away it will be weaker.
So bigger atoms (towards the bottom of the group) with the outer orbital far from the nucleus will loose their electron more easily: this means they react more easily/quickly/more/vigorously.
Smaller atoms with the electron closer to the pull of the nucleus (at the top of group one) will be less reactive as it takes more to lose the electron.
Group one elements need to loose an electron- the one on the outer shell- to react. Electrons are held to an atom by the protons in the nucleus. If an electron is close to the nucleus the force holding it in will be very strong, if it is further away it will be weaker.
So bigger atoms (towards the bottom of the group) with the outer orbital far from the nucleus will loose their electron more easily: this means they react more easily/quickly/more/vigorously.
Smaller atoms with the electron closer to the pull of the nucleus (at the top of group one) will be less reactive as it takes more to lose the electron.
2.6 describe the reactions of these elements with water and understand that the reactions provide a basis for their recognition as a family of elements
The group one elements- lithium, sodium, potassium- are easily identifiable as the same group due to the fact that they all react vigorously with water (clearly due to the fact they have similar electronic configurations.)
The reactions that occur are huge- they get bigger further down the group. Hydrogen gas is produced as well as metal hydroxide.
Search on youtube; there are plenty of videos of there reactions.
The reactions that occur are huge- they get bigger further down the group. Hydrogen gas is produced as well as metal hydroxide.
Search on youtube; there are plenty of videos of there reactions.
2.5 understand that the noble gases (Group 0) are a family of inert gases and explain their lack of reactivity in terms of their electronic configurations.
Nobel gasses are inert, this means they do not react. The reason for this is because they are stable: meaning they have a full outer shell, so they do not need to loose or gain electrons.
2.4 understand why elements in the same group of the Periodic Table have similar chemical properties
Elements in the same group have the same number of electrons on their outer shell.
This means they will behave in a similar way; they will react and bond similarly.
For example in group one, the elements all form+ 1 ions as they each loose one electron to become stable (to have a full outer shell).
This means they will behave in a similar way; they will react and bond similarly.
For example in group one, the elements all form
2.3 explain the classification of elements as metals or non-metals on the basis of their electrical conductivity and the acid-base character of their oxides
Metals are all conductors. Metals form metal-oxides which are alkaline.
Nonmetals don't conduct. They form nonmetal-oxides which are acidic.
Nonmetals don't conduct. They form nonmetal-oxides which are acidic.
2.2 recall the positions of metals and non-metals in the Periodic Table
 |
| bbc |
To the left are the metals, to the right are the nonmetals
2.1 understand the terms group and period
Groups are columns in the periodic table, the number of a group represents the number of electrons on an atom's outer shell.
Periods are rows on the periodic table, the row represents the number of orbitals that a atom has.
Periods are rows on the periodic table, the row represents the number of orbitals that a atom has.
1.56 recall that one faraday represents one mole of electrons
One Faraday is 96500 coulombs. That is the amount of coulombs in one mole of electrons.
Subscribe to:
Posts (Atom)

